Iron Metabolism
Contents:
Many mechanisms involved in the regulation of hepcidin synthesis in relation to iron have been elucidated [ 25 , 26 , 27 ]. Physiological and pathological conditions such as release of bone morphogenetic protein BMP [ 28 ], hypoxia [ 29 , 30 ] as well as endocrine [ 31 , 32 , 33 , 34 ], metabolic [ 35 , 36 ], and inflammatory [ 17 , 37 , 38 ] processes modulate hepcidin biosynthesis and may therefore regulate availability of iron to erythropoiesis by adaptation of iron absorption and recirculation. Many mechanisms are involved in the regulation of hepcidin synthesis.
The peptide is mainly produced by the liver, in responses to many different mechanisms. In presence of inflammation as well as in situations with increased intracellular and extracellular iron stores, the concentration of hepcidin is increased. Inversely, when iron requirements are high, such as in increased erythropoiesis, hepcidin levels are low. Hepcidin blocks the exportation of iron from hepatocytes, macrophages as well as from the enterocytes, by binding to ferroportin FPN1 allowing it internalization and degradation illustrations used elements from Servier Medical Art: Iron sensing is dependent on an external pathway implicating the interactions of Tf on Tfr1 and Tfr2 and aid by the protein HFE.
The binding of iron-loaded Tf to Tfr1 followed by the binding of Tfr2 depends on iron saturation of Tf; if iron-Tf is high, the Tfr2-mediated signaling by the BMP6 receptor complex is increased. In contrast, hepcidin mRNA is suppressed in anemia [ 59 ], but this effect is probably indirect, depending on the erythropoietin production [ 60 ]. The first protein is hemojuvelin HJV a glycosylphosphatidylinositol-linked membrane protein [ 41 ], the second is Mt2 [ 42 ], which regulates the levels of membrane-bound HJV, and the third is neogenin, a ubiquitously expressed transmembrane protein with multiple functions [ 43 , 44 , 45 , 46 , 47 , 48 ].
The gene of Mt2 carries several polymorphisms that have been linked to iron metabolic parameters, notably in patients presenting with iron-refractory iron-deficient anemia [ 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 ]. In blood, hepcidin exists in mature- and pro-hormone form prohepcidin. Prohepcidin was found to specifically bind to the STAT3 site in the promoter of the HAMP gene, thus suggesting that prohepcidin affects the expression of its own gene, indicating an autoregulatory loop of hepcidin gene expression [ 24 ]. Using liquid chromatography in combination with high-resolution mass spectrometry, we and others were able to identify new forms of hepcidin in human plasma or serum samples [ 59 , 60 ].
Iron is present in many different types of cells, having specific functions such as iron supply or iron storage. Iron-exporting cells include enterocytes, which absorb iron from the digested food, macrophages and hepatocytes, which both recycle iron according to demand. In addition, placental syncytiotrophoblast cells transport iron into the fetal circulation. IRPs bind to iron-responsive elements IREs located in the untranslated regions of genes and mRNAs encoding proteins involved in iron uptake, storage, utilization, and export. Males contain about 4, mg of iron, of which 2, mg are within erythrocytes; 1, mg is stored in splenic and hepatic macrophages, and the rest is distributed in various proteins such as Mb, cytochromes, or other ferroproteins.
Only about 3 mg are bound to plasma Tf and constitute the mobile iron compartment which supplies the various intracellular iron stores. Iron metabolism is finely regulated. Males contain about 4, mg of iron, of which 2, mg is within erythrocytes; 1, mg is stored in splenic and hepatic macrophages, and the rest is distributed in various proteins such as myoglobin, cytochromes or other ferroproteins.
About 1—2 mg of iron is lost every day, through skin and enteric desquamation and minor blood losses. This loss is balanced by intestinal absorption. Therefore, iron recycling accounts for most of the iron homeostasis in human. The situation is different in menstruating women where there are discussions about iron stores, ferritin and hemoglobin levels illustrations used elements from Servier Medical Art: The situation is different in menstruating women [ 62 , 63 ] where there are controversial discussions about iron stores, ferritin, and Hb levels [ 64 , 65 ].
It appears that lower Hb and ferritin values in menstruating women have been accepted as normal rather than possibly representing widespread iron deficiency.
Hepcidin expression in the liver: Hepatic uptake of iron and iron sensing are afforded by TfR1 and transferrin receptor 2 TfR2; Figure The Journal of Clinical Investigation. N Engl J Med. Widespread population based screening is difficult to justify due to the relatively low phenotypic penetrance and low prevalence of mutations in certain ethnic groups. There is a hinge between the C1 and C2 domains of the C-lobe and also a hinge between the N1and N2 domains of the N-lobe. Hepcidin as a biomarker for the diagnosis of iron metabolism disorders:
The situation is even more complex in pregnant women; nevertheless, iron substitution has been shown to be beneficial for them [ 66 , 67 ]. Similarly, increased iron demand occurs during infancy and childhood due to growth and development demands [ 68 , 69 , 70 ]. Iron is the most abundant element on earth, with potential of high toxicity to living cells. However, it has poor bioavailability, and efforts have been made to provide iron for everybody, notably by food fortification within rice [ 71 ], because it represents one of the most essential nutriments for human beings.
Rice and most staple cereals contain low iron levels, since most iron-containing components are lost during grain processing. Populations with monotonous diets consisting mainly of cereals are especially prone to iron deficiency, which affects about two billion people. Food fortification programs to supplement nutrition with iron have not been very successful.
One alternative solution is iron biofortification. Different approaches have been studied, including conventional breeding and directed genetic modification, which offer the most rapid way to develop iron-rich rice plants [ 71 ]. Biofortification of crops is also an interesting approach [ 72 ], and at least two complementary approaches have been successfully adopted to increase the concentrations of bioavailable mineral elements in food crops.
Secondly, crops have been developed with increased abilities to acquire mineral elements and accumulate them in edible tissues. In the same context, it seems necessary to highlight the efforts made by some investigators, who developed high-iron rice, using transgenic approaches. They created high-iron rice by insertion of soybean ferritin gene under the control of the endosperm-specific glutelin promoter into the genome of the Indica rice line [ 73 ]. However, and because of widespread skepticism about transgenic food, it is still necessary to know the iron content of the usual food taken by our populations.
This is why the knowledge of the iron content of various aliments as well as of the factors influencing its absorption should be improved [ 74 ]. Finally, from a hematologist point of view, universal iron fortification of the food may be problematic, notably for individuals with hemochromatosis and other iron loading diseases [ 75 ]. Even if iron fortification of food has been recognized by some authors as a suitable strategy to combat iron deficiency, some health authorities have abandoned it.
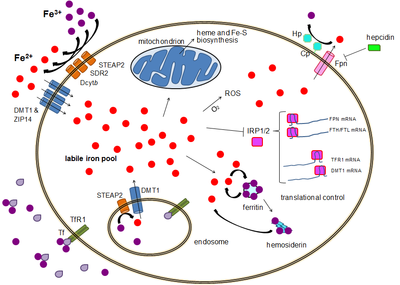
Readers interested in iron fortification, iron food, and other deviancies are referred to the recent reviews published in [ 67 , 76 ]. Iron absorption is the result of complex mechanisms that takes place in the upper parts of the gut, notably in the duodenum and the proximal jejunum [ 16 , 77 ] fig. Regulation of iron absorption and exportation by enterocytes.
Both heme and non-heme iron are absorbed by specific pathways, including divalent metal transporter-1 DMT-1 and heme carrier protein HCP1 , in association with the ferrireductase, duodenal cytochrome B Dcytb. Within the cell, iron can be stored within the ferritin molecule. The metal is exported by the protein ferroportin FPN1 , and transported into the blood by transferrin. In presence of hepcidin, ferroportin is internalized and degradated. Thus, iron exportation is blocked. Inversely, in the absence of hepcidin, ferroportin is maintained on the cell membrane, and iron transportation is facilitated illustrations used elements from Servier Medical Art: Non-heme iron is associated with various storage proteins, including ferritin, whereas heminic iron is present within hemoproteins such as Mb or Hb.
It is important to note that non-heme iron is captured by several complexes which can interfere with its absorption, notably plant-derived phytates or tannins [ 78 ]. Ascorbic acid and other acidic components derived from the diet can increase iron absorption. Nevertheless, it is known that different pathways exist for the absorption of non-heme iron and heme iron. The distinction is of potential interest, because it has been shown that high heme iron intake leads to increased body iron stores which are significantly associated with higher risk to develop type 2 diabetes mellitus [ 79 ].
In contrast, total dietary iron, non-heme iron, and intake of iron supplements were not associated with type 2 diabetes mellitus. Several well regulated gate keeper proteins are expressed in the duodenum enterocytes and are differently regulated as compared to the same proteins in liver cells. Of note, ferric reductase activities due to duodenal cytochrome B [ 82 ] and STEAPs six transmembrane epithelial antigen of the prostate proteins [ 83 ] are present on the brush border of duodenum allowing reduction of ferric to ferrous iron, thus facilitating its absorption by DMT1.
Heme iron is an important nutritional source of iron in carnivores and omnivores that is more readily absorbed than non-heme iron derived from vegetables and grain. Most heme is absorbed in the proximal intestine, with absorptive capacity decreasing distally, and the role of specific proteins such as hephaestin has been deciphered [ 84 , 85 ]. HCP1, which presents homology to bacterial metal-tetracycline transporters, mediates heme uptake by the cells at the luminal brush border membrane of duodenal enterocytes. Once iron is present in the enterocyte, its fate de pends on the iron pool within the cell.
Iron has to be exported from cells to the circulation, and a specific protein, FPN1, has been identified in this function. FPN1 is a multipass protein found in the basolateral membrane of the enterocytes. Furthermore, FPN1 is the unique iron export membrane protein that is present in large quantities on macrophages. Over-expression of FPN1 is induced by cellular iron, and it is suppressed by hepcidin. Hepcidin binds to cell surface FPN1 inducing its internalization which is followed by lysosomal degradation [ 21 ].
Thus, as a consequence, the iron efflux from enterocytes or macrophages is suppressed, leading to reduced iron absorption by duodenal enterocytes. Deletion of the FPN1 gene results in a complete block of iron exportation associated with accumulation of the metal within enterocytes and macrophages [ 86 ]. Without activity of ferroxidases, FPN1 is internalized and degraded [ 87 , 88 ]. Thus, the ferroxidases at the cell surface mediate stability of FPN1. In humans with aceruloplasminemia, anemia is associated with impaired cellular iron export [ 89 ].
As previously mentioned, HCP1, which is also a ferroxidase, has also an important role during iron export from intestinal enterocytes and its subsequent loading to Tf. Structurally, the ectodomain of HCP1 resemble Cp [ 90 ]. Tf is the main protein involved in iron transport in plasma. The diagnostic value of Tf has just been reviewed [ 91 ].
It proved to be a useful parameter for assessing both iron deficiency and iron overload. The saturation of Tf is a strong indicator of iron overload. However, from a physiological point of view, the iron binding capacity of plasma Tf is often exhausted, with concomitant generation of non-Tf-bound iron NTBI as observed in transfused patients. Using fluorescent tracing of labile iron in endosomal vesicles and cytosol, Kloss-Brandstatter et al.
Erythrocyte precursors restrictively take up iron by using Tfr, notably Tfr1, whereas hepatocytes and other non-erythroid cells are also able to use NTBI. Iron-Tf binds to Tfr, and the complexes are internalized within the cell by the endosomal recycling vesicles. Thus, the Tf cycle is dependent on the Tf-Tfr complex trafficking, involving internalization of the complex within endosome, followed by iron release upon acidification of the endosome and recycling of the Tf-Tfr complex to the cell surface. Each of these steps is mediated by a specific pathway and specific machinery [ 93 , 94 , 95 ].
Finally, at the cell surface, at neutral pH, Tf dissociates from Tfr, and is used to repeat the iron cycle. In addition, Tfr is cleaved and shed as a soluble form sTfr into the extracellular and intravascular space. This shedding of Tfr1 is known for more than 30 years, and its assessment is well accepted as a diagnostic marker of iron-depleted erythropoiesis [ 96 , 97 , 98 ].
Very recently, the cleavage site as well as the cleaving proteases of membrane Tfr1 have been identified [ 99 ]. Only ferric iron is transported to the cytoplasm or to mitochondria. It is therefore mandatory to reduce ferrous irons; a family of ferrireductase has been identified. DMT1 is also an essential protein involved in iron transportation from vacuole into the cytoplasm [ ].
In macrophages, another protein Nramp1 is involved [ , ]. Due to its toxicity, iron within the cytoplasm is associated with proteins such as poly RC -binding protein 1 [ ], functioning as cytosolic iron chaperone in the delivery of iron to ferritin. Within the ferritin molecule, iron is stored in the ferric form associated with hydroxide and phosphate anions [ ]. Each ferritin molecule can sequester up to approximately 4, iron atoms. Ferritin also has enzymatic properties, converting ferric to ferrous iron, as iron is internalized and sequestered in the ferritin mineral core.
Small quantities of ferritin are also present in human serum and are elevated in conditions of iron overload and inflammation. De Domenico et al. An interesting observation was made by Mikhael et al. For decades, serum ferritin has been used for assessing iron disorders, and its value as a marker of body iron has been recently reviewed [ ].
Several genetic alteration of ferritin genes have been reported [ ], notably in association with a specific neurological disease [ ]. Erythroid precursors need much more iron than any other type of cells in the body, and, as previously mentioned, they take up iron almost exclusively through Tfr1. Iron transport into mitochondria is provided by mitoferrin-1, the mitochondrial iron transporter 1 of erythroid precurors [ ]. Mitoferrin-1 interacts with an ATP-binding transporter and binds to ferrochelatase to form an oligomeric complex [ ], allowing iron uptake and heme biosynthesis.
Erythroid cells contain adaptative mechanisms to face iron deficiency and a class of kinases activated by different cellular stresses. HRI-deficient mice have allowed identifying HRI as a protector of apoptosis and being involved in the formation of microcytes. Several groups reported on the genetic polymorphism of the proteins involved in iron homeostasis, but not related to iron deficiency or overload [ , , ]. Genetic analysis of iron deficiency in mice has been evaluated [ ]. This study revealed that polymorphisms in multiple genes cause individual variations in iron regulation, especially in response to dietary iron challenge.
In humans, genome-wide association studies found linkage of various gene polymorphism single nucleotide polymorphism; SNP and iron status, notably polymorphism of the gene coding for Mt2 [ 56 , , , , ]. Other investigators showed an association between Mt2 polymorphism and the risk to develop type 2 diabetes [ 52 ].
The authors observed that individuals homozygous for iron-lowering alleles of Mt2 had a reduced risk of iron overload and of type 2 diabetes. In a genome-wide association study looking at heme iron uptake polymorphisms, no significant association with type 2 diabetes and iron metabolic pathways were identified [ ].
In an analysis of several genes modulating iron status, Pelucchi et al. Iron is a key player in hemoglobin synthesis an erythrocyte production. At the same time, it is a potent poison to mammalian cells and an indispensable nutrient for many disease-causing germs and microbes. Therefore, its metabolism in mammalians is very complex and stringently controlled by many different genes and proteins. Identification of the genes and their polymorphic alleles may shed light into the metabolic interplay of relevant proteins.
BF also received research grants from Vifor Pharma. National Center for Biotechnology Information , U. Journal List Transfus Med Hemother v. Published online May Author information Article notes Copyright and License information Disclaimer. Received Oct 8; Accepted Dec 4. This article has been cited by other articles in PMC.
Summary A revolution occurred during the last decade in the comprehension of the physiology as well as in the physiopathology of iron metabolism. Iron, Metabolism, Transfusion medicine. Introduction Various tests have been developed to evaluate iron metabolism and iron stores, and nowadays bone marrow examination has been replaced by the measurement of blood ferritin [ 1 ]. Iron Metabolism and Proteins The physiology of iron trafficking and metabolism has been well evaluated over the last 20 years, and several comprehensive reviews have been published on the subject [ 16 , 17 , 18 , 19 , 20 , 21 , 22 ].
Open in a separate window. Iron Regulatory Proteins Iron is present in many different types of cells, having specific functions such as iron supply or iron storage. Iron in the Body Males contain about 4, mg of iron, of which 2, mg are within erythrocytes; 1, mg is stored in splenic and hepatic macrophages, and the rest is distributed in various proteins such as Mb, cytochromes, or other ferroproteins.
Iron in the Food; Unusual Aspects Iron is the most abundant element on earth, with potential of high toxicity to living cells. Intestinal Iron Exportation Once iron is present in the enterocyte, its fate de pends on the iron pool within the cell. Iron Transportation in Blood and Import Tf is the main protein involved in iron transport in plasma. Intracellular Iron Storage Only ferric iron is transported to the cytoplasm or to mitochondria. Iron and Erythropoiesis Erythroid precursors need much more iron than any other type of cells in the body, and, as previously mentioned, they take up iron almost exclusively through Tfr1.
Genetic Polymorphism of Proteins Involved in Iron Metabolism Several groups reported on the genetic polymorphism of the proteins involved in iron homeostasis, but not related to iron deficiency or overload [ , , ]. Conclusions and Perspective Iron is a key player in hemoglobin synthesis an erythrocyte production.
Laboratory diagnosis of iron-deficiency anemia: J Gen Intern Med. Screening for hereditary haemochromatosis. TFR1 has a fold higher affinity for transferrin-bound iron than TFR2 and thus is the main player in this process. Iron from this pool can be taken up by mitochondria via mitoferrin to synthesize Fe-S clusters and heme groups.
Iron can be stored in ferritin as ferric iron due to the ferroxidase activity of the ferritin heavy chain. Iron export occurs in a variety of cell types, including neurons, erythrocytes, macrophages and enterocytes. The latter two are especially important since systemic iron levels depend upon them. There is only one known iron exporter, ferroportin.
Introduction
The expression of hepcidin, which only occurs in certain cell types such as hepatocytes , is tightly controlled at the transcriptional level and it represents the link between cellular and systemic iron homeostasis due to hepcidin's role as "gatekeeper" of iron release from enterocytes into the rest of the body. Although some control exists at the transcriptional level, the regulation of cellular iron levels is ultimately controlled at the translational level by iron-responsive element-binding proteins IRP1 and especially IRP2.
Both ferritin and ferroportin contain an IRE in their 5' UTRs, so that under iron deficiency their translation is repressed by IRP2, preventing the unnecessary synthesis of storage protein and the detrimental export of iron. Functional or actual iron deficiency can result from a variety of causes. These causes can be grouped into several categories:. The body is able to substantially reduce the amount of iron it absorbs across the mucosa. It does not seem to be able to entirely shut down the iron transport process. Also, in situations where excess iron damages the intestinal lining itself for instance, when children eat a large quantity of iron tablets produced for adult consumption , even more iron can enter the bloodstream and cause a potentially deadly syndrome of iron overload.
Normal Iron Metabolism and the Pathophysiology of Iron Overload Disorders
Large amounts of free iron in the circulation will cause damage to critical cells in the liver, the heart and other metabolically active organs. Iron toxicity results when the amount of circulating iron exceeds the amount of transferrin available to bind it, but the body is able to vigorously regulate its iron uptake. Thus, iron toxicity from ingestion is usually the result of extraordinary circumstances like iron tablet over-consumption [1] [35] rather than variations in diet.
The type of acute toxicity from iron ingestion causes severe mucosal damage in the gastrointestinal tract, among other problems. Excess iron has been linked to some cancers. Of note, a recent study showed that breast cancer patients with low ferroportin expression leading to higher concentrations of intracellular iron survive for a shorter period of time on average.
Chronic iron toxicity is usually the result of more chronic iron overload syndromes associated with genetic diseases, repeated transfusions or other causes. Classic examples of genetic iron overload includes hereditary hemochromatosis HH and the more severe disease juvenile hemochromatosis JH caused by mutations in either the gene RGMc gene, a member of a three gene repulsive guidance molecule family, [37] also called hemojuvelin HJV , and HFE2 , Hemojuvelin , or the HAMP gene that encodes an iron regulatory peptide.
The exact mechanisms of most of the various forms of adult hemochromatosis, which make up most of the genetic iron overload disorders, remain unsolved. So while researchers have been able to identify genetic mutations causing several adult variants of hemochromatosis, they now must turn their attention to the normal function of these mutated genes. From Wikipedia, the free encyclopedia. The New England Journal of Medicine. Free Radical Biology and Medicine. Applied and Environmental Microbiology. Advanced Nutrition and Human Metabolism 6th ed. Archived from the original on June 16, Retrieved June 25, The Journal of Biological Chemistry.
Biochimica et Biophysica Acta. Key molecules and mechanisms and their roles in disease".
Seminars in Pediatric Neurology. Journal of Cell Science. Annual Review of Nutrition. Scand J Gastroenterol Suppl. Explicit use of et al. Metallomics and the Cell. Metal Ions in Life Sciences. The Journal of Clinical Investigation. Camaschella C Dec Intestinal iron absorption and its regulation".
American Journal of Physiology. Gastrointestinal and Liver Physiology. Jones and Bartlett Publishers. Handbook of Nutrition and Pregnancy. Food and Nutrition Board, Institute of Medicine. The Nutritional Trace Metals. Metabolism , catabolism , anabolism. Metabolic pathway Metabolic network Primary nutritional groups. Pentose phosphate pathway Fructolysis Galactolysis. Photosynthesis Anoxygenic photosynthesis Chemosynthesis Carbon fixation. Fatty acid degradation Beta oxidation Fatty acid synthesis. Steroid metabolism Sphingolipid metabolism Eicosanoid metabolism Ketosis Reverse cholesterol transport.
Amino acid synthesis Urea cycle. Purine metabolism Nucleotide salvage Pyrimidine metabolism. Metal metabolism Iron metabolism Ethanol metabolism. Phosphoric acids and phosphates.
- Colonising Myths – Maori Realities: He Rukuruku Whakaaro!
- Section outline!
- Jede Menge Glück (German Edition)!
- Physiology of Iron Metabolism.
Calcium-sensing receptor Calcium-binding protein. Retrieved from " https: Iron metabolism Hematology Human homeostasis Biology and pharmacology of chemical elements. Uses authors parameter CS1 maint: Views Read Edit View history.
This page was last edited on 10 December , at By using this site, you agree to the Terms of Use and Privacy Policy. Electron acceptors are other than oxygen.