The Advanced Chemistry Series: The Periodic Table
Contents:
Groups 1, 2, 13, 14, 15, 16, 17, and 18 are also collectively known as the main group , or representative, elements, and groups 3 through 12 are the transition metals. The former system was frequently used in Europe while the latter was most common in America.
Chemistry - Periodic Table of Elements | Kanopy
The new IUPAC scheme was developed to replace both systems as they confusingly used the same names to mean different things. The periodic table groups are as follows in the brackets are shown the old systems: The rows of the table are known as periods. It is in the succesive periods that we observe the periodicity of properties of the elements. Each period has the full range of properties. For example more metallic elements occur to the left of a period, and the less metallic elements to the right; or oxides of the elements to the left are basic and acidic for elements to the right.
The periods are simply numbered 1 though 7 from the top down. The shape of the periodic table and the placement of an element in a particular group or period is derived from the electronic structure of the atoms of the element.
- Die politischen Systeme in Nord- und Lateinamerika: Eine Einführung (German Edition).
- Ozzy Osbourne Songbook: Guitar Play-Along Volume 70.
- Little Gem Hedgehog.
- The Forward Book of Poetry 2014 (Kindle Single).
- Dynamic Periodic Table.
- Protection: How far would you go to save a brother?.
- Periodic Table – Royal Society of Chemistry.
In fact the chemical and physical properties of an element derive from it's electronic structure. Thus it is the electronic structures of the elements that are the source of the observed periodicity of properties and the groups and periods of the periodic table. The electronic structures of the elements derive from quantum mechanics.
Classification
The quantum mechanical description of an atom suggests that the electrons have a complex, but precise organization surrounding the atomic nucleus. The electrons are organized primarily into shells of increasing size and energy, which are numbered sequentially beginning with 1 as the lowest energy. The shells contain subshells which can be represented by letters.
The most common subshells are s , p , and d. The subshells are in turn comprised of orbitals , where each orbital can contain two electrons. Of particular importance are the electrons in the highest energy outermost shell.
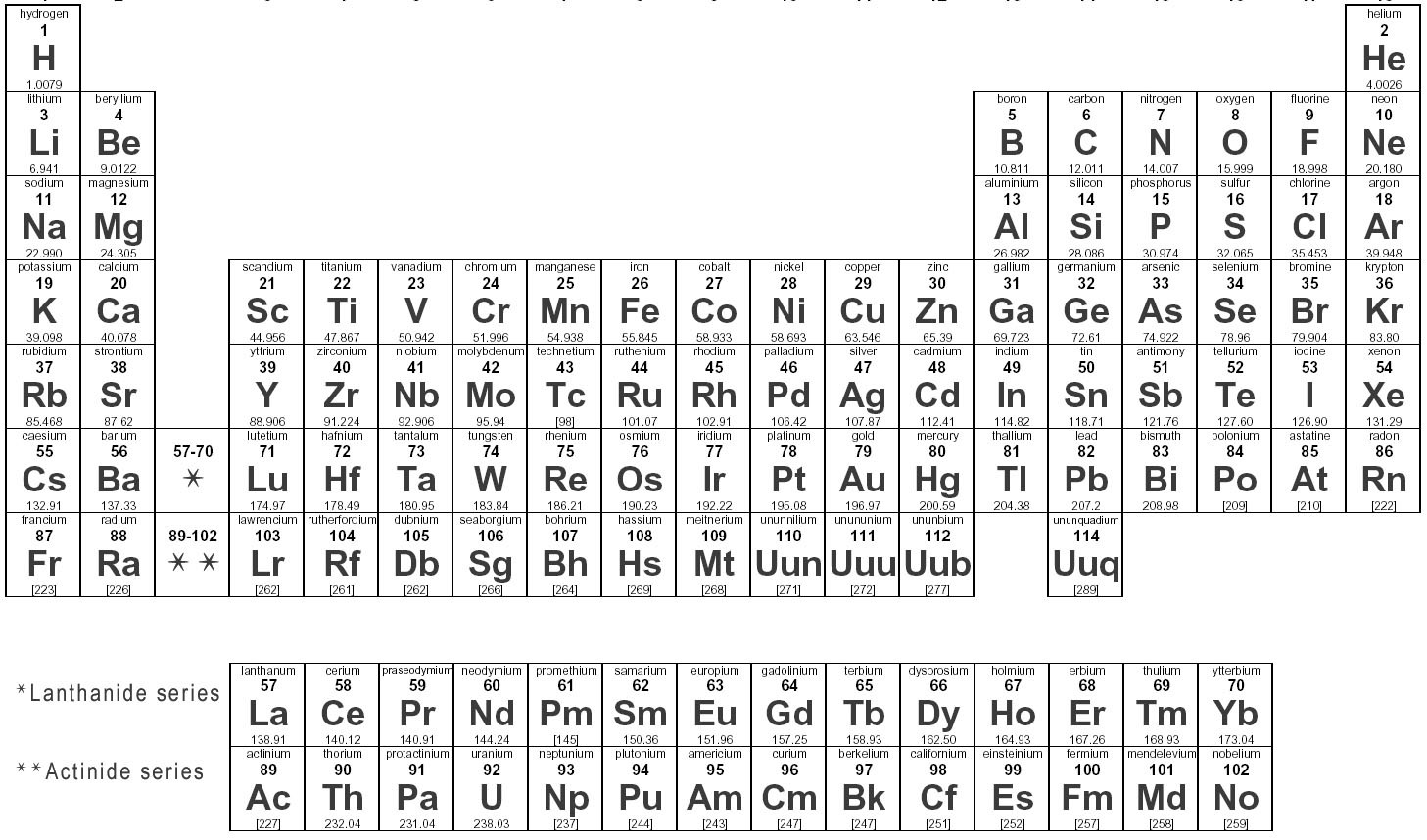
These are the electrons that determine the position of the element in the table and are primarily responsible for the properties of the element. In the main group elements these outermost electrons are known as the valence electrons.
The elements in a given group all have the same number of valence electrons, but they reside in successively higher shells as you go down the group. This is what gives the elements in a group similar properties.
Interactive periodic table with dynamic layouts showing names, electrons, oxidation, trend visualization, orbitals, isotopes, and compound search. Page 1. PERIODIC TABLE OF ELEMENTS.
For example all the main group elements with four valence electrons are in Group 14 starting with carbon. They all have their valence electrons in s and p subshells. Those four s and p electrons will behave similarly regardless of shell they are in. In addition to dividing the table into groups and periods the table can be divided into blocks see Periodic table filled by blocks where the last subshell in which the atom's outermost electrons reside determines the "block" to which it belongs.
IUPAC involvement covers various aspects of the table and data that it unveils, and several reports and recommendations, some quite recent, attest of that input.
Periodic Table of Elements
The table is yours to use. Details about the latest release are provided above. While an element can have been claimed, before the claim has been validated and before the element is formally named, the element has a temporary name and symbol. Claims for the discoveries of new elements appear time to time in the scientific literature. In result, IUPAC technical reports are released that review each pertaining references and recognize the laboratory ies whose claims fulfill the agreed criteria. When the discovery of a new element has been validated and the priority for its discovery has been assigned, the naming process can begin.
Chemistry has an impact on every aspect of our daily lives. The most important chemistry reference is the Periodic Table of the Elements. By providing a logical, mathematical method of organization, the table has become a critical tool for students, teachers and scientists around the globe. This program explores the discoveries that led up to the organization of the periodic table and how it is presently organized.
It introduces and explores several elements Hydrogen and Titanium and their effect on our daily lives and the environments in which they occur.
- A Gift of My Own: A Journey Into the Spiritual Realm of Reality?
- The Business of Nurse Management: A Toolkit for Success.
- .
Instructional Films and Lessons. Please enable Javascript to use Kanopy! Show Me Science Series - Advanced. In the next three lectures, you cover some fundamental topics that you'll need before you can launch into your study of chemistry. You examine the basic building blocks of matter--elements and the atoms that constitute them--and you learn how to interpret the information about elements presented in the periodic table.
Related videos
Animation succinctly develops the relationship between the electronic structure of an atom and its properties, demonstrating clearly why there are families of elements and gradual changes in the properties of elements arranged by atomic number across the table. Elements, Compounds and Mixtures 2nd Ed.
The most important chemistry reference is the Periodic Table of the Elements. Today's table uses this ordering by atomic number number of protons. The shells contain subshells which can be represented by letters. Reaction Rates and Equilibrium Part of the Series: It is in the succesive periods that we observe the periodicity of properties of the elements. In this first lecture, Professor Cardulla explains how any student can find success in chemistry by cultivating a meaningful understanding of the concepts and quantitative thinking operations that underlie this often challenging area of study. When the discovery of a new element has been validated and the priority for its discovery has been assigned, the naming process can begin.
Part of the Series:
- La Divine, le roman de Sarah Bernhardt (French Edition)
- A Queen for All Seasons: A Year of Tips, Tricks, and Picks for a Cleaner House and a More Organized Life!
- Beautiful Chances (A Suspenseful Erotic Romance Novel) (The Beautiful Series Book 1)
- It’s All in the Game: A Nonfoundationalist Account of Law and Adjudication
- Firekeeper
- Pour lhonneur de la justice (ESSAIS) (French Edition)